Table of Contents
- What is the Meaning of KKDIK?
- What is the Purpose of KKDIK?
- Differences and Similarities Between KKDIK and EU REACH
- The Importance of KKDIK for Local and Foreign Companies
- Key Requirements of KKDIK (Turkish REACH)
- KKDIK Compliance Process
- KKDIK Registration Fees
- KKDIK Deadlines
- Common Challenges in KKDIK Compliance and How to Overcome Them
- How We Support Your KKDIK Compliance
- Frequently Asked Questions
What is the meaning of KKDIK?
KKDIK is the harmonized version of the EU REACH Regulation into Turkish chemical legislation. It stands for “Kimyasalların Kaydı, Değerlendirilmesi, İzni ve Kısıtlanması Hakkında Yönetmelik” which translates to “Regulation on the Registration, Evaluation, Authorisation, and Restriction of Chemicals” as in the REACH. Like EU REACH, KKDIK places certain obligations for companies to protect human health and the environment from the hazards of chemicals.
One of these obligations is KKDIK registration. Substances not registered under KKDIK cannot be manufactured or imported in Turkey, which means KKDIK covers not only Turkish companies but also non-Turkish companies.
Understanding KKDIK
What is the purpose of KKDIK?
As the KKDIK Regulation is a harmonization of the EU REACH Regulation, they have similar purposes. In KKDIK Regulation, its purpose is stated in Article 1 below:
“The purpose of this Regulation is to regulate the administrative and technical procedures and principles regarding the registration, evaluation, authorisation and restriction of chemicals to ensure a high level of protection of human health and the environment, to encourage alternative methods for the evaluation of the hazards of substances, and to increase competition and innovation.”
With these purposes, the KKDIK Regulation puts companies that manufacture, import or use certain chemical substances under some obligations.
Differences and Similarities Between KKDIK and EU REACH
Although KKDIK and EU REACH regulations have much in common, they also have some differences. These similarities and differences can be seen in the table below:
| Aspect | KKDIK (Turkey REACH) | EU REACH | Similarity | Difference |
| Scope | Applies to chemicals manufactured or imported into Turkey. | Applies to chemicals manufactured or imported into the EU. | Both cover the manufacture, import, and use of chemicals in their respective regions. | KKDIK only applies to Turkey, while REACH applies to all EU member states. |
| Legal Framework | Based on Turkish Regulation No. 30105, enacted in 2017. | Based on Regulation (EC) No 1907/2006, enacted in 2006. | Both have national/regional legislative frameworks. | KKDIK is newer and reflects updates to align with REACH but with Turkey-specific legal requirements. |
| Language Requirements | All documentation and submissions must be in Turkish. | All documentation must be in an official EU language, primarily English. | Both require submissions in the official language. | Language requirement is Turkish for KKDIK, and an official EU language (often English) for REACH. |
| Authority | Ministry of Environment, Urbanization and Climate Change (Turkey). | European Chemicals Agency (ECHA) manages the process. | Both have dedicated authorities to oversee regulations. | Different authorities: KKDIK is overseen by Turkey’s Ministry, while REACH is managed by the EU’s ECHA. |
| Registration Deadline | Registration deadlines extended until 2030 for chemicals in Turkey. | The last phase-in deadline for REACH was in 2018. | Both have phased deadlines for chemical registration. | KKDIK has more recent deadlines, giving Turkish companies more time compared to REACH’s earlier timeline. |
| Data Sharing | Requires data sharing to reduce duplicate testing, but compliance processes may vary slightly. | Strong emphasis on data sharing within the EU, specifically to minimize animal testing. | Both prioritize data sharing to avoid duplicate testing. | KKDIK may have slight differences in how data-sharing agreements are implemented. |
| Chemical Evaluation | The evaluation follows a similar structure but is adapted to Turkish conditions. | Evaluations are carried out centrally by ECHA with input from member states. | The evaluation process is similar for both regulations. | Evaluation authority differs: centralized at ECHA for REACH, localized for KKDIK. |
| Authorization Process | Follows a similar authorization process for SVHCs (Substances of Very High Concern) but with adaptations for Turkey. | Requires authorization for SVHCs across the EU. | Both regulate SVHCs through an authorization process. | KKDIK has Turkey-specific adaptations, while REACH has broader EU-based authorizations. |
| Restriction Measures | Restrictions based on Turkish laws and evaluations, mirroring REACH’s approach. | Restrictions set under Annex XVII of REACH, apply across the EU. | Both restrict chemicals which risk uncontrolled | KKDIK restrictions may include Turkey-specific evaluations, while REACH restrictions cover the entire EU. |
| Compliance and Enforcement | Turkish authorities handle enforcement, with penalties for non-compliance. | EU member states’ national authorities enforce REACH, guided by ECHA. | Both have strict enforcement and penalties. | Different enforcement bodies: Turkey for KKDIK, national authorities with ECHA’s guidance for REACH. |
According to the table above, the key differences between KKDIK and EU REACH are language requirements, deadlines, and authorities. However, they both serve the same purpose: chemical safety, which leads to certain similarities.
The Importance of KKDIK for Local and Foreign Companies
Importance for Foreign Companies
One of the most important benefits of KKDIK is to achieving Turkish market access. Companies that wish to export their products into Turkey are expected to apply for KKDIK registration in order to gain legal access to the Turkish market. In case of non-compliance with the KKDIK Regulation, foreign companies cannot take place in the market. Besides, achieving compliance with the KKDIK Regulation will be helpful for compliance with EU REACH too, as they have many similarities.
As you know, the main purpose of KKDIK is to ensure chemical safety. That’s why, achieving compliance with KKDIK is a responsibility for companies, both local and foreign. As this is a responsibility for other companies as well, foreign companies can benefit from data-sharing agreements which help them to reduce costs and to avoid redundant testing.
Lastly, assuring KKDIK compliance will avoid significant penalties such as product bans, restrictions, and legal fines. It will also help the companies to maintain a strong presence in the Turkish market and to improve their reputation.
Importance for Local Companies
As stated above, KKDIK compliance is necessary for companies that wish to have a presence in the Turkish market. In addition to that, as KKDIK and REACH have many similarities, a company that is compliant with KKDIK also fulfils a large part of international chemical safety standards.
In their KKDIK compliance process, companies share data with other registrants, which helps them to reduce the cost of testing and access necessary data easily. In Turkey, the number of small businesses is quite high. KKDIK data-sharing helps these businesses with their financial burden of compliance.
Another benefit of KKDIK for local companies is to fulfil their responsibility towards safety and the environment in the context of chemicals. This legal compliance helps them to gain a reputation and trust both within Turkey and globally. It also leads to maintaining good industry practices and securing long-term sustainability.
As KKDIK compliance is a legal obligation, non-compliance with it will lead to legal penalties such as product recalls, restrictions or fines. By achieving compliance with KKDIK, companies can avoid these negative consequences as well.
Key Requirements of KKDIK (Turkish REACH)
- KKDIK pre-registration and registration
- Data-sharing
- Authorization
- Notification
- Communication in the supply chain
- Classification and labelling
- Safety data sheets
- Duty to substitute with safer alternatives
- Restrictions
KKDIK Compliance Process
- Information Requirements
According to the Guidance on Registration by the Turkish Chemicals Help Desk, manufacturers and importers must collect information on the substance that they manufacture or import. They must use this information for risk assessment and management to ensure chemical safety.
Information to be collected includes but not limited to:
• Data from in vitro and in vivo tests,
• Non-test data obtained from alternative methods such as (Q)SAR •(Quantitative) Structure-Activity Relationship, grouping of substances and read-across,
• Information on manufacture, use and risk management measures and resulting exposure.
It should be noted that precise information requirements for each substance may vary based on tonnage, use and exposure as well as available information on intrinsic properties. In cases where available data is not adequate, additional tests may be required. - KKDIK Registration Dossier
KKDIK Registration dossier is the data set that is submitted by a registrant for a certain substance. A KKDIK registration dossier consists of two parts: a technical dossier and a chemical safety report (CSR).
Technical Dossier
A technical dossier is a document that is always required for substances subject to registration obligations.
A technical dossier includes information on:- Identity of the manufacturer or importer,
- Identity of the substance,
- Information on the manufacture and use of the substance,
- Classification and labelling of the substances,
- Guidance on safe use,
- Study summaries on the intrinsic properties of the subtance,
- Robust study summaries on the intrinsic properties of the substance, if required
- Whether notification that the manufacture and use, classification and labelling, comprehensive study summaries and/or, if relevant, the chemical safety report has been reviewed by an assessor,
- Recommendations for further testing, if relevant,
- Exposure information for substances present in quantities between 1 and 10 tonnes,
- Request, including justification as to what information will be considered confidential.
Chemical Safety Report (CSR)
The chemical safety report is the documentation of the registrant’s chemical safety assessment (CSA). CSA and CSR obligations apply if the 10 tonnes per year threshold is met. However, there are some exceptions, which must be determined by an expert by assessing the substances.
Format of KKDIK Registration Dossier and Submission
According to the KKDIK Regulation, registrations must be conducted via the Chemical Registration System, known as KKS. The format of the registration dossier is set by the system itself. Submission of the dossier is also done via this system.
- Data Sharing Procedures
In the case of where a substance has more than one manufacturer or importer, they must submit certain information together. This is called “joint registration”, applies to all substances.
According to the KKDIK Regulation Article 12, registrants must submit information on the intrinsic properties of the substance (if any study and testing proposals exist) and classification and labelling information, and if they agree on this, a CSR and guidance on safety. If the company is based outside of Turkey, the only representative must take part in joint registration.
As the data sharing process can be quite challenging, it is recommended to get outside support such as KKDIKPro, a data sharing platform.
KKDIK Registration Fees
KKDIK Registration fees are updated every year and published on the Ministry’s website. To learn about KKDIK registration fees in 2024, you can check out the article titled “2024 KKDIK Registration Fees Have Been Published”.
These fees are determined based on tonnage, existence of SME discount, discount for joint submission if available and confidential information.
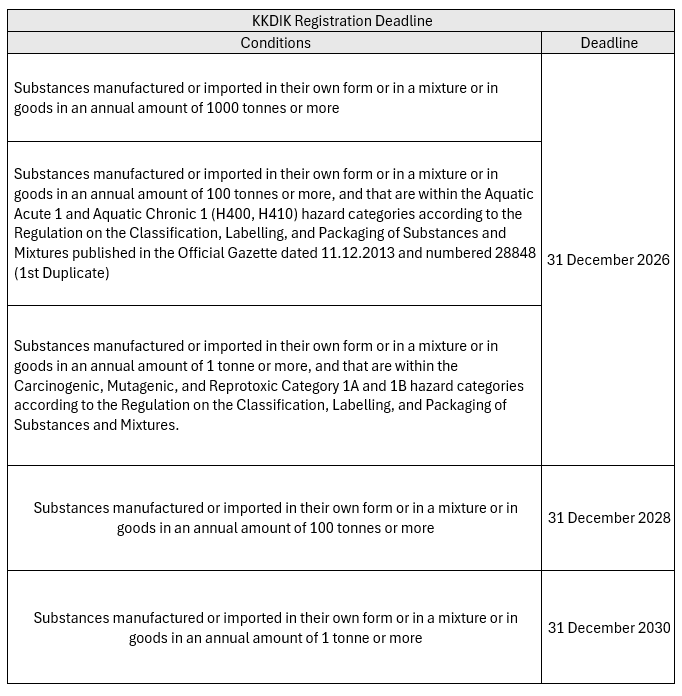
KKDIK Deadlines
Deadlines for KKDIK are as below:
Common Challenges in KKDIK Compliance and How to Overcome Them
KKDIK compliance process is quite complex. Achieving a successful compliance process both requires technical and legal knowledge. As companies generally do not have this kind of expertise in-house, it can be hard for them to navigate the process smoothly.
The most common challenges that companies face include:
- Information overload
- Compliance issues – not having up-to-date information
- Inexperience in the use of KKS
- Language barriers
- Security and confidentiality
- Time management
Details and tips to overcome these challenges are stated in our article named “Challenges in KKDIK Registration”.
To avoid these challenges and conduct a timely compliance project, you can benefit from our support.
How We Support Your KKDIK Compliance
In your KKDIK compliance process, we can help with:
- Determination of the company role and responsibilities
- Management of the full process for substances
- Determination of chemical characterization of the substances
- Data management and analysis
- Pre-registration process
- Pre-SIEF and SIEF participant activities
- Consortium activities
- Translations, preparation, compilation, and submission of the registration dossier
- Chemical Safety Report authoring
- Establishment of the substance inventory list under Annex-17.
For more information regarding KKDIK compliance and our services, you can contact us.
Frequently Asked Questions
KKDIK Regulation is a Turkish regulation that aims to ensure chemical safety. It is an adaptation of the EU REACH Regulation.
Companies that manufacture or import chemical substances in Turkey are under the scope of the KKDIK registration obligation.
What information will be made available to the public after KKDİK registration?
The information below will be made available to the public:
a) IUPAC name,
b) EINECS name of the substance, if any,
c) Classification and labelling of the substance,
d) Data on physical-chemical properties and movement and behaviour within the environment
e) Toxicological and ecotoxicological information,
f) DNEL or PNEC,
g) Guidance on safe use,
h) Analytical methods for the detection of a hazardous substance in the environment and determination of human exposure information, if requested in accordance with KKDIK Annex-9 or Annex-10.
