Table of Contents
What is REACH Registration?
REACH stands for “Registration, Authorisation, and Restriction of Chemicals (REACH)”. It is a regulation that aims to minimize the adverse effects of chemicals on human health and the environment.
According to the REACH Regulation, manufacturers and importers must provide detailed information about the chemicals they produce or import. This information should be submitted through the European Chemicals Agency (ECHA). This is essential for companies working with chemicals in the European market to ensure compliance and chemical safety. REACH covers a wide range of products including:
- Furniture
- Kitchen utensils
- Electronics
- Textiles
- Packaging materials
Guidance on REACH Registration
What is the aim of REACH Registration?
According to ECHA’s Guidance on REACH Registration, the main aim of it is to ensure that manufacturers, importers and downstream users use, manufacture or place on the market substances that are safe for human health and the environment. That is why natural or legal persons who take part in the manufacture, import, place on the market or use of such substances are held responsible for REACH compliance.
The REACH Registration is a means to ensure that natural or legal persons in question meet the related obligations.
How to do REACH Registration?
REACH Registration is preparing a registration dossier in IUCLID format and submitting it to ECHA via the REACH-IT system. According to ECHA, the registration process can be divided into five steps:
- Information requirements;
- REACH registration dossier;
- Joint submission of data;
- Confidentiality and electronic public access to registration information;
- Access to documents.
These steps are discussed below.
- Information Requirements
The first step of the registration process involves obtaining the necessary information. Manufacturers and importers are required to submit the related data which will be used to assess the risks and take precautions if necessary.
This information is expected to be involved in the REACH registration dossier. In the process of obtaining this information, some tests and analyses may be required, which must comply with GLP principles. - REACH Registration Dossier
A registration dossier consists of two main components: a technical dossier and a chemical safety report. The details of each are stated below:
- Technical dossier
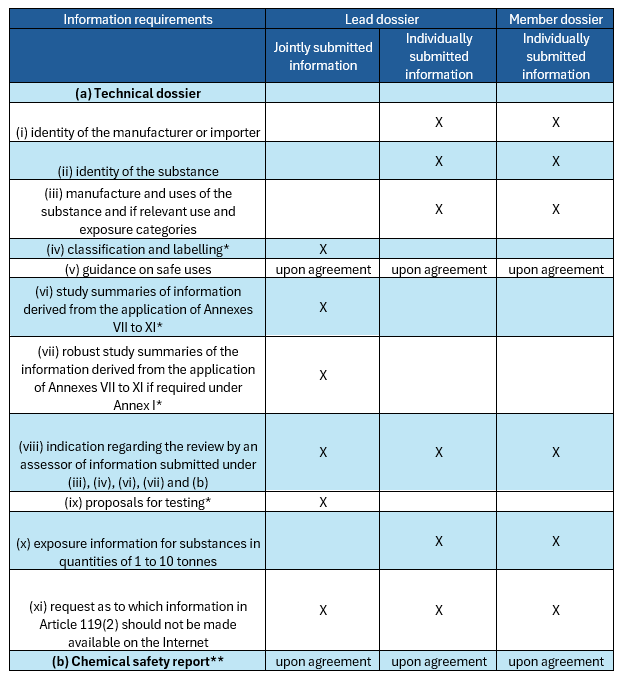
According to the ECHA’s Guidance on REACH Registration, a technical dossier is always required for all substances subject to registration obligations.
It includes information on:- The identity of the manufacturer/importer;
- The identity of the substance;
- Information on the manufacture and use of substance;
- The classification and labelling of the substance;
- Guidance on its safe use;
- Study summaries of the information on the intrinsic properties of the substance;
- Robust study summaries of the information on the intrinsic properties of the substance, if required;
- An indication as to whether the information on manufacture and use, the classification and labelling, the (robust) study summaries and/or, if relevant, the chemical safety report has been reviewed by an assessor;
- Proposals for further testing, if relevant;
- For substances registered in quantities between 1 and 10 tonnes, information on exposure;
- A request as to which information should be considered confidential, including a justification.
- Chemical Safety Report
Substances that are manufactured or imported by the registrant in quantities of 10 tonnes or more per year are subject to chemical safety assessment (CSA) requirements. After CSA is completed, it must be documented in a chemical safety report, known as CSR.
However, there are some exemptions. According to ECHA, a CSR is not required for a substance present in a mixture if the concentration of the substance in the mixture is less than the lowest of the values defined in Article 14(2). In addition, for uses in food contact and cosmetics, the chemical safety report is not required to address human health as it is addressed under other legislation.
- Technical dossier
- Joint Submission of Data
In cases where multiple individuals wish to register the same substance, they must take part in a joint submission. According to ECHA, the joint submission includes a discussion of the data, sharing its costs, and submitting the information that is required under Articles 11(1) and 19(1) of REACH jointly.
This way, the costs of registration are minimized, and duplication of the test is avoided. These registrants can also benefit from reduced registration fees.
The three cases regarding information in a joint submission are summarized in the table below:
| Registrants are required to submit jointly the following information | Registrants may decide to submit jointly or separately | Registrants must submit separately in their dossier |
| Classification and labelling of the substance | Guidance on the safe use of the substance | Their identity |
| (Robust) study summaries and proposals for testing | Chemical safety report (CSR) when required | The identity of the substance |
| Indication as to which of the submitted information on classification and labelling study summaries and robust study summaries have been reviewed by an assessor chosen by the registrant and having appropriate experience | Indication as to which of the information submitted for the CSR has been reviewed by an assessor | Information on the manufacture and uses |
| Exposure information for substances in quantities of 1 to 10 tonnes | ||
| Indication as to which of the information on manufacture and use has been reviewed by an assessor |
In a joint submission, there are two types of registration dossiers: lead dossier and member dossier.
According to ECHA, the lead dossier involves information on the lead registrant and the data set required in REACH for the highest tonnage band covered by the jointly submitted data. Member dossier, on the other hand, is the dossier that each of the registrants of the joint submission must submit individually.
Below, there is a table including details of the dossiers:
4. Confidentiality and electronic public access to registration information
In addition to ensuring chemical safety, the REACH Regulation also ensures the safety of information held by ECHA. According to ECHA’s Guidance on Registration, such information shall be either disclosed upon request or made publicly available on the ECHA website.
Guidance on how to request confidentiality in registration may be found on the ECHA’s manual page with the title ‘Dissemination and confidentiality under the REACH Regulation”.
5. Access to documents
ECHA states that documents held may be granted based on a case-by-case assessment as it is foreseen in ATD Regulation. According to ATD Regulation, ECHA must consult the author of the document on whether it should or should not be disclosed.
EU REACH Registration Fees
The REACH registration process incurs certain fees, which vary based on the substance’s tonnage and type of registration. Companies need to plan their budget accordingly to avoid non-compliance penalties. Late registrations may result in financial penalties, making timely action crucial. We advise companies not to delay their registration process to avoid extra costs. For information regarding REACH registration fees for your substances, you can contact us.
Restricted Substances Under REACH
REACH Regulation places certain restrictions for chemicals, heavy metals and pollutants in all products. Products that are restricted under REACH cannot be imported and sold within the single market legally. This application aims to prevent the dangerous use of chemicals and ensure safety against hazardous substances.
Substances that are restricted may include lead, phthalates, cadmium and mercury. All of them are stated in the ECHA’s Restricted Substances List.
Achieving REACH Compliance
By achieving the steps above correctly, you can ensure legal compliance with the REACH Regulation. If you don’t have the necessary expert workforce in-house, it is recommended that you retrieve some outside support from trustful consultancy companies.
We provide comprehensive support to companies in the chemical industry, ensuring a smooth and accurate REACH registration process. Our experienced team handles the preparation of the required technical dossiers and offers expert guidance at every step, ensuring quick and correct completion of the process.
Ensure your company’s REACH obligations are met with ease by partnering with us. We offer expert support to help you maintain compliance and gain a competitive advantage in the market.
Contact us now for more information and to start your registration process.
Frequently Asked Questions
Registration in REACH is the process of submitting information about the chemical substances that wish to be manufactured or imported to the European Chemicals Agency.
REACH registration obligations apply for companies that manufacture or import chemical substances into the European Union market.
Actually, there is not a certification that implies REACH compliance. However, REACH compliance can be demonstrated through registration dossiers, safety data sheets and labelling.
There is not a certification scheme for REACH compliance. Similarly, there is no such thing as a REACH certificate. Registration dossiers, SDSs and labelling can be used for compliance demonstration.
Products that contain chemical substances and that are wished to be manufactured or imported into the EU market may be subject to REACH Regulation. Such products cover a wide range of areas such as industrial chemicals, electronic products, textiles etc.
It is not possible to state a certain duration for the REACH registration process. However, it can take a few months to several years depending on the complexity of the substance, data quality and cooperation with authorities.
It is not possible to state an exact estimate of the REACH testing costs as it varies depending on the substance details and necessary tests. It is recommended to get a quote from an expert to obtain a correct estimation of the costs.
